The periodic table example is often taken into account while preparing it for any purpose. It is basically a table of chemical elements, where all periods as well as groups are organized either in a horizontal or vertical column or row with respect to expending atomic number. We can say in other words as it is a tabular form of representation of all elements present in-universe by the way of periodic manner. All elements in this table are arranged according to the periodic law, which states the elements with similar properties are needed to be arranged in horizontal rows called groups and vertical columns called periods. The aim of using this table is quite simple, as to gain information about any element regarding its atomic mass and chemical symbol. As far as, its history is concerned, a large development was seen in the field of analytical chemistry during 19th century, where many new compounds and elements were discovered which developed the need for their proper classification. To cater this deficiency, J.W. Döbereiner was the first scientist who proposed the idea of triads in which elements were classified in the group of three in which middle elements have atomic weight same to the average atomic weight of a first and third element.
Classification of a Periodic Table:
The idea of J. W Döbereiner was quickly recognized by many scientists and later, John Alexander Reina Newlands suggested that all elements should be categorized as per their increasing atomic weight. But after the finding of valance (that is every atom capacity to form a bond with other atoms), a Russian chemist, Dmitri Mendeleev identify elements in increasing order of their atomic masses and therefore, made the first ever periodic table in the year 1869. Afterwards, New Zealand-born British physicist Ernest Rutherford conducted multiple experiments and his famous experiment which concluded us to the finding of atomic number. It was known that elements have different atomic masses but the same atomic number (number of protons) in the nucleus. These series of research and hard-work done in 19th century resulted on which the basis of modern periodic table is come into reality.
Periodic Table as a Tool:
As elements are arranged in specific order, a periodic table gives us the idea about atomic mass, number, and properties of any element and which elements are chemically and physically related. It can easily be used as a reference for many other known and unknown elements. All branches of chemistry, including physical analytical organic-inorganic and biochemistry, use this table as a reference. Furthermore, it is used by students and scientists to determine the nature of any kind of chemical reaction inside or outside within its environment. It tells us about the electron acceptor or donor elements, which is the most important phenomena on which electricity is based.
Periodic Table on Experimental Purposes:
During the 19th century, Ernest Rutherford identified the concept of a radioactive element’s half-life which can be established by looking at the table and can help in understanding their nature, can be used in conducting wide dimensional experiments. The experiments of making of a hydrogen bomb (H bombs) or the nuclear fusion reactions, involves the first understanding of the periodic table. A periodic table gives the basic idea about ionization, electronegativity and metals metalloids nature which are now part of this table. There was much development seen in chemistry-related fields, after the formation of periodic table. Now, minor flaws are to be seen as it gives both general and comprehensive view about every aspect of chemistry and other about other basics sciences so far.
Benefits of Using a Periodic Table:
It is clear that periodic table helps us in understanding chemistry a lot better, such as with the help of periodic table, we understand the properties of an element better if we are aware of its position in it, and if we know it’s position, it also helps us in knowing what type of compound the element will form. Also, it has the benefit of the table being based on atomic numbers as it is an essential property. Periodic table are easy to note and can be repeated. Metal and non-metals are separated in the period table, which helps you in differentiating them better.
Templates for Periodic Table:

 sciencenotes.org
sciencenotes.org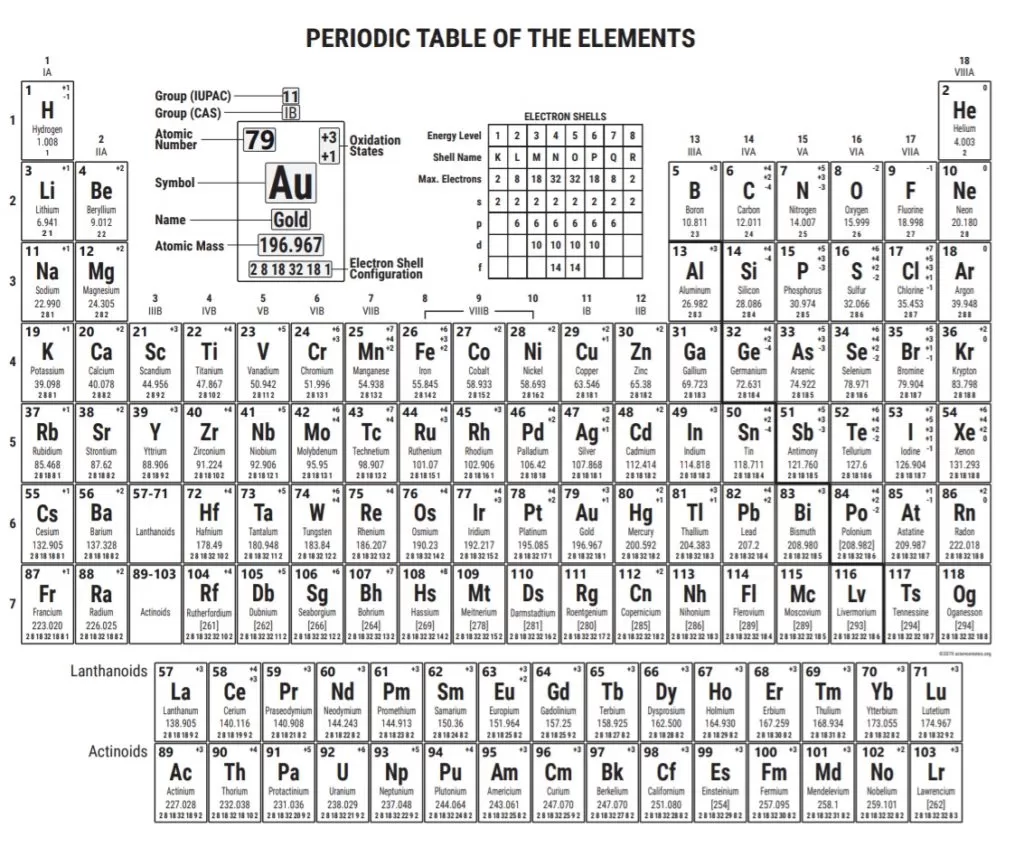
 sciencenotes.org
sciencenotes.org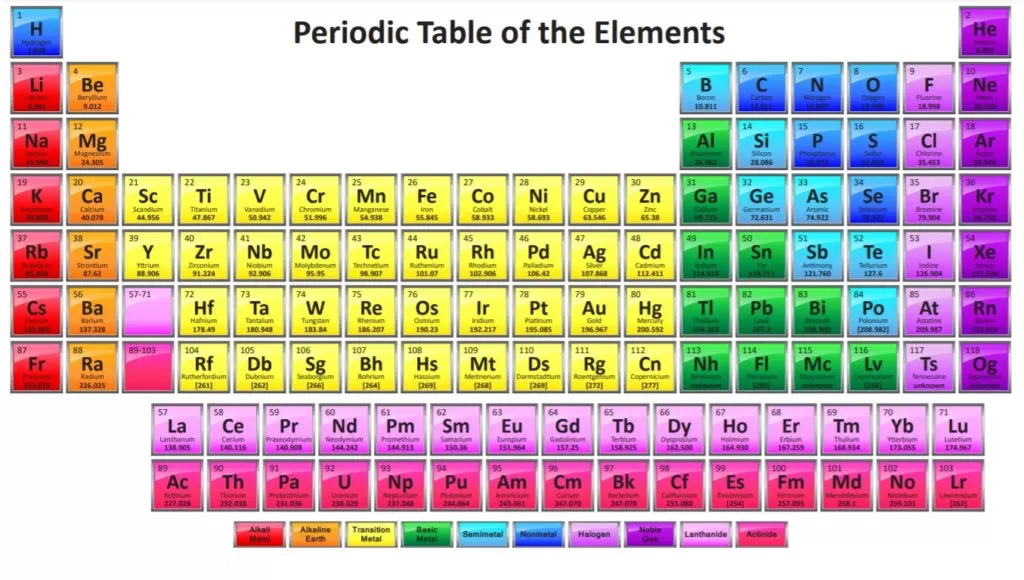
 sciencenotes.org
sciencenotes.org
 www.thoughtco.com
www.thoughtco.com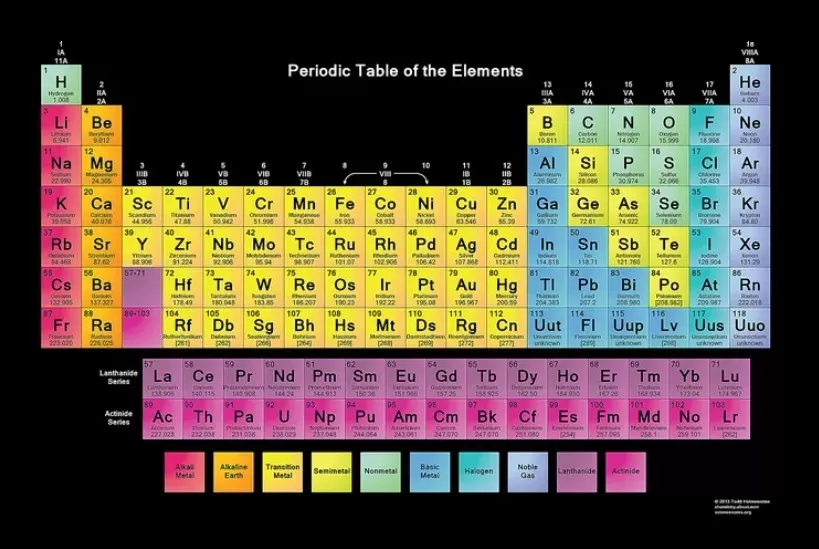
 www.thoughtco.com
www.thoughtco.com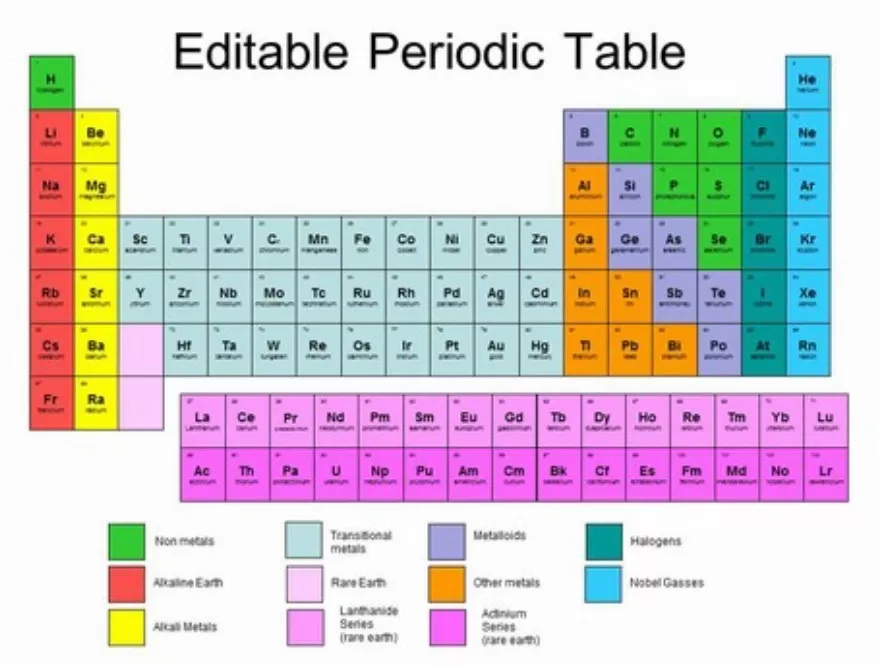
 www.presentationmagazine.com
www.presentationmagazine.com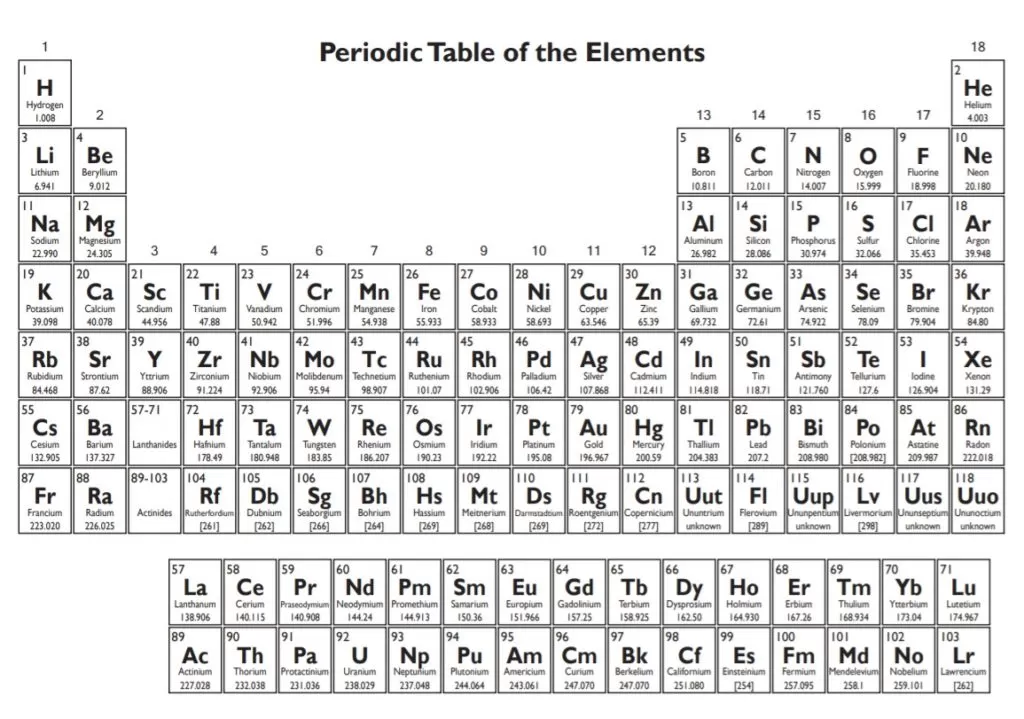
 neprisstore.blob.core.windows.net
neprisstore.blob.core.windows.net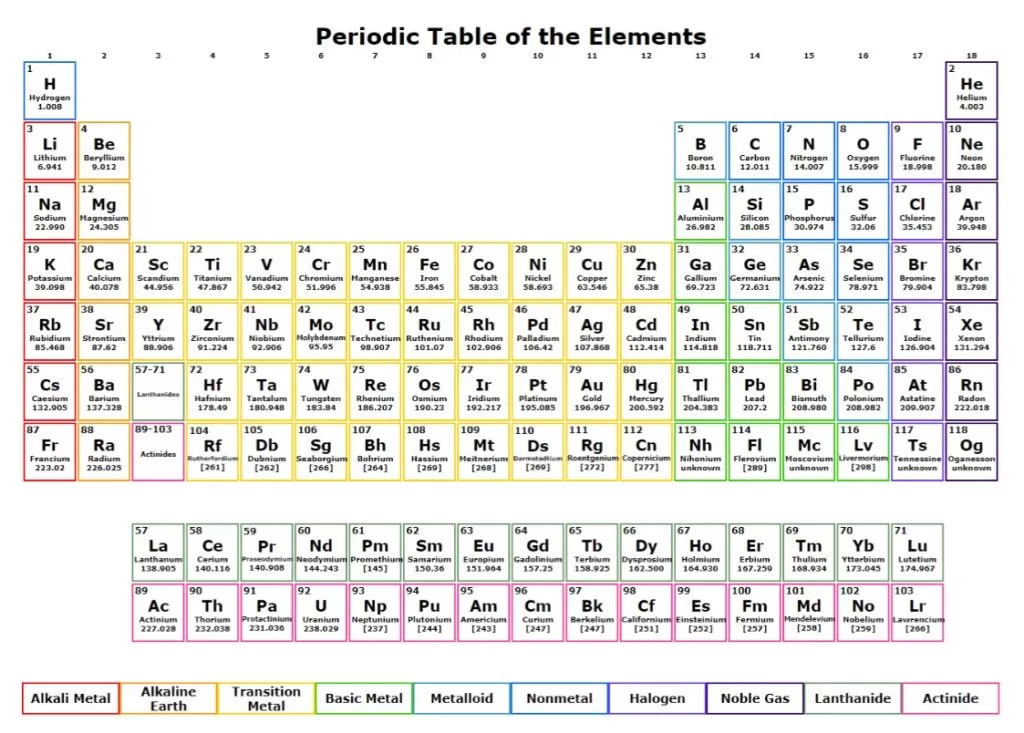
 handypapertemplates.com
handypapertemplates.com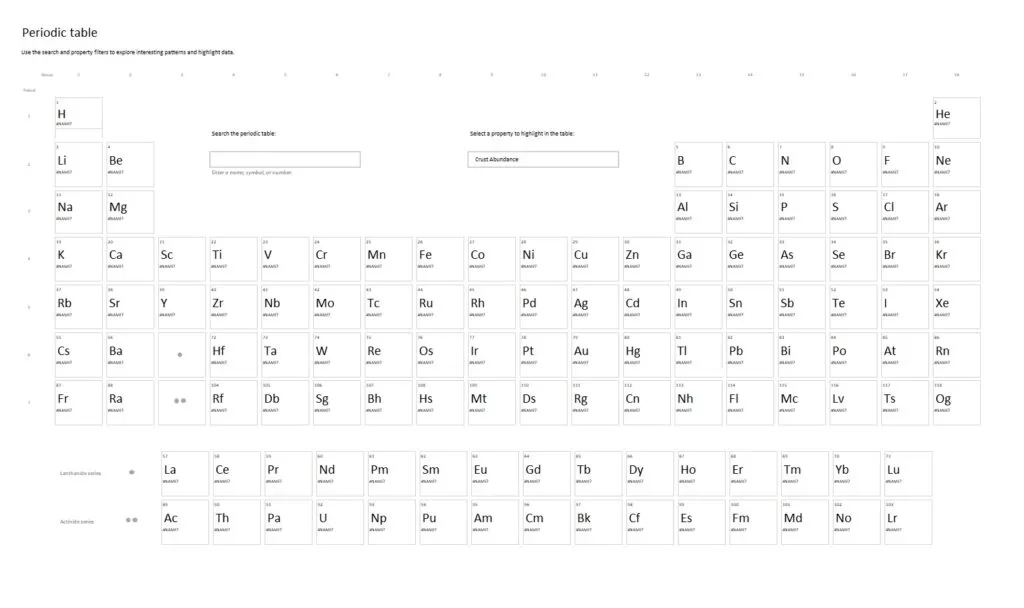
 exceldownloads.com
exceldownloads.com